Report a non-conformance
The improvement register enables you to non-conformance with operational systems or processes. After an non-conformance is reported, it moves to the Manage stage where the actions required to respond to the suggestion will be determined.
Reporting a nonconformance provides insights into where existing controls to manage a process or product needs to be strengthen. A nonconformance is a failure of a system, process, or activity to conform to its stated purpose or objective.
Quick steps to suggest a non-conformance
- Go to the Improvement register, click the FAB and select Improvement
- Select 'Non-conformance' from the Improvement type menu
- Complete the Improvement details form
- Click Submit
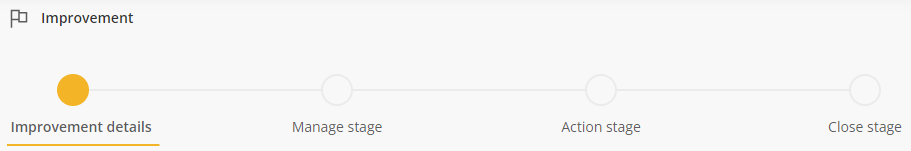
Workflow
The Stage Navigation Bar displays the workflow stages that the improvement will follow once added (subject to the decisions made along the way).
Form components
| Improvement details | Describes and categorises the improvement. |
| Non-conformance details | Where you describe the non-conformance |
| Assign | Where you can assign who the improvement is reported to. |
| Attach records | Where you can upload or link related records to the reported improvement. |
| System event history | A chronological list maintained by the system of most entries, changes, and linkages made in the system in relation to this contract. |
Improvement details
This component enables you to describe the underlying issue and the recommended improvement.
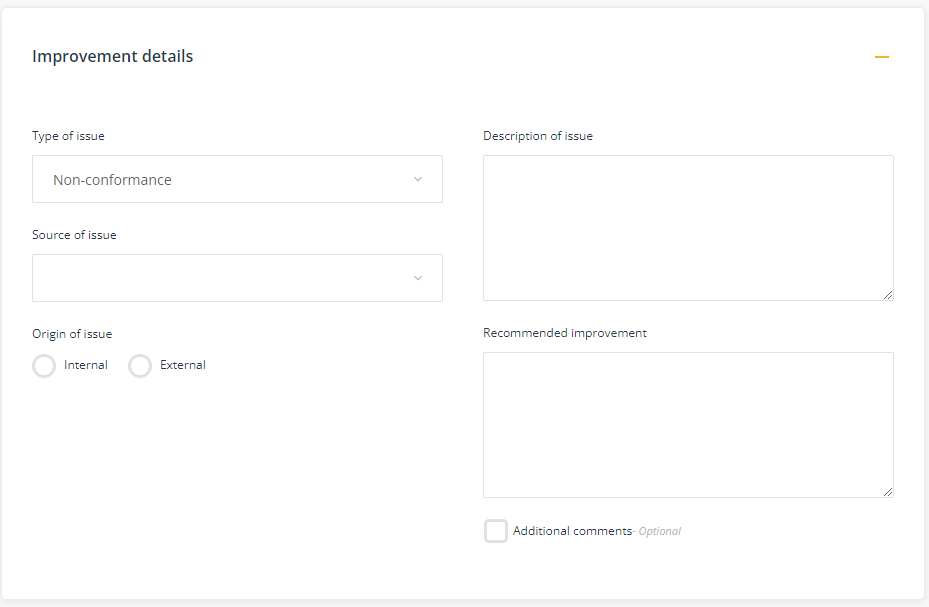
- Type of issue - select 'Non-conformance' (see Suggest an improvement for suggesting opportunities for improvement).
A non-conformance is where a system, process or activity doesn't meet its intended purpose. The non-conformance could be because there has been a failure to follow the procedures, or because the documented procedures are inadequate, for example.
- Source of issue - select the source of the non-conformance. The source could be through an adverse event occurring, for example, a customer complaint, a work health and safety incident or other adverse event, damage to equipment,
- Origin of issue - select where the issue was identified: internal (within the organisation) or external (outside of the organisation, eg external auditor). If the improvement was identified by an external source, specify the name of the person or the company name.
- Description of issue - describe the non-conformance eg. 'procedures for security were not followed'; 'equipment wasn't delivered as per the agreed time'. Avoid making comment about what the solution is at this stage.
Note: this field is limited to 2000 characters. Use the Attach records to attach further information, eg documents, emails, photographs. - Recommended improvement - make a recommendation as to how the process or activity could be better managed in order to avoid or reduce the chance of the non-conformance occurring again in the future.
Non-conformance details
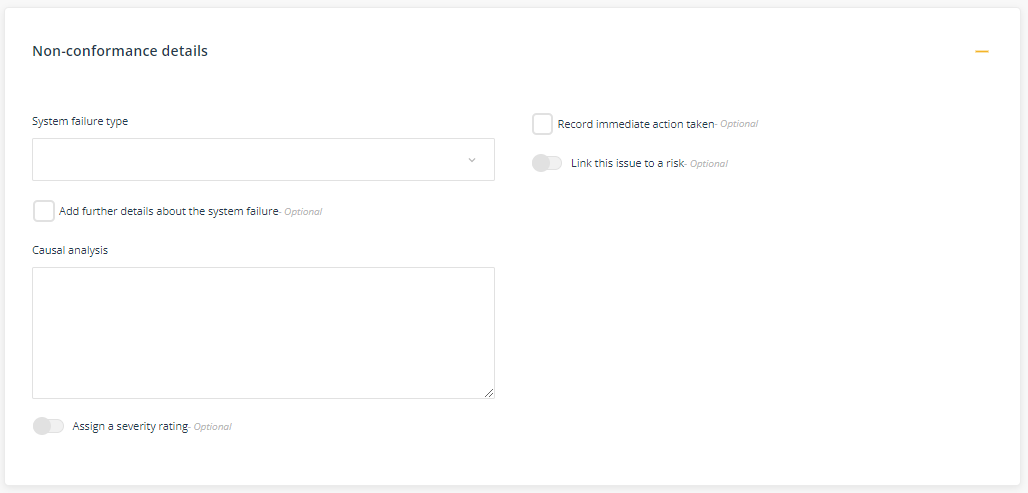
In selecting Non-conformance' as the Type of issue to be reported, an additional component on the Improvement Details form will open. The Non-conformance details component prompts you to give consideration as to why the non-conformance occurred though undertaking a causal analysis (cause-and-effect).
- System failure type - select the business system that most likely led to the failure. For example, where the failure related to new staff not following procedures select 'Employee Induction Program'.
- Add further details about the system failure (optional) - if you want to make additional comments, tick this box to open an additional text box field.
- Causal analysis - In describing the causal analysis (cause-and-effect) try to identify the underlying reason why the issue occurred. At this stage, continue to stay away from trying to find the solution. A useful tool in conducting a causal analysis is to use the '5 Whys'. Here is an example of using the '5 whys' to identify the underlying cause as to why the clinic wasn't able to vaccinate a client today:
- Why - because we didn't have the required vaccine
- Why - because we didn't place the order on time last week
- Why - because the person who normally orders the vaccine was away on leave and the task wasn't allocated to another staff member
- Why - because there is no management oversight for this task
- Why - because the position responsible for oversight is vacant
Based on this example, you would suggest that the underlying cause of the non-conformance is due to the vacancy in the position with responsibility for oversight in this area.
- Record immediate action taken (optional) - document details of what action has been taken in response to the non-conformance. For example, 'I immediately followed up on the order for the vaccines and confirmed date of delivery'.
- Link this issue to a risk (optional) - select from the list of identified risks, the relevant risk. For example, if there is an identified risk relating to 'management of medical consumables' it would be relevant to link the non-conformance relating to the un-availability of vaccinations to the identified risk, 'management of medical consumables'.

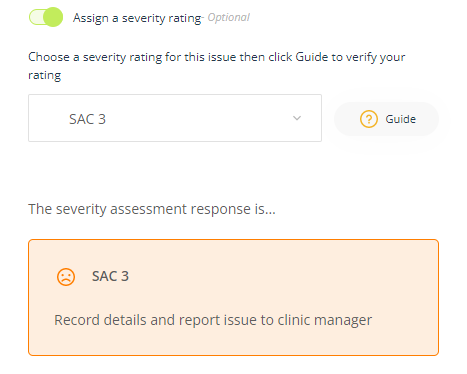
Severity rating
A Severity rating is a numerical score that rates the severity of an adverse event.
- Assign a severity rating (optional) - This option will also display at the Manage stage.
To assign a severity rating, toggle the Assign a severity rating button and select an option from from the list.
Click the help Guide to assess whether you have selected the option that best fits the severity of the non-conformance. To understand the difference between the severity levels, select each one and read the notes in the Guide. This sometimes helps to ensure you have chosen the correct rating. - The severity assessment response will display when you select a rating - The severity assessment response provides a guide as to what the organisation's standard response protocols are in managing an event based on the severity level that has been selected.
Assign
This component enables you to specify who the improvement is reported to and which business area the improvement relates to ensure delegations and communication relating to the management of the improvement aligns with organisational requirements.
- Related business area - select the business area that the non-conformance relates to.
- Related meeting - select the team meeting who is responsible for monitoring this type of non-conformance or select the team meeting that is be kept informed of this type of non-conformance. For instance, if the process for ordering medical consumables is the responsibility of the clinical team then there would be a benefit of adding the non-conformance to the Clinical Team Meeting. This is a good way of reminding staff of the importance of ensuring procedures are followed.
- Report this issue to - select the staff member who is responsible for managing this type of non-conformance. The person selected will be notified immediately of the non-conformance and will be assigned the task to manage the feedback.
- Notify other users by email (optional) - You can use this function to notify relevant personnel that the non-conformance has been added to the QMS, thereby providing them with a link to the non-conformance. Note: users sent notifications relating to the non-conformance will need to be included in Access control to see the non-conformance.
Access control
The Access control component is where you define which users have access to view the item. You can grant access to a team or individual users, or both.
| All users can access | Select this option if you would like all users to be able to view the item. |
| Specify who can access | Select this option to control which users can view this item. |
| Teams | Select the team/s who need to view the item. |
| Individual users | Select specific users (if they are not included in the selected team/s) who need to view the item. |
| Who can view? |
Click this button to see a list of users who can see the item based on your selection. The list will also include those users assigned to the item in the workflow. Note: Users with system level permissions to see all items will also be included. |
Important: If your organisation has implemented the Business Rules Builder feature, some or all of the assign fields may be pre-populated and not editable. These selections will be based on conditional logic that has been pre-coded by your System Administrator. This feature helps to ensure the non-conformance is assigned to the right business area, right meeting, right manager, right viewing permission levels, and the right people have been notified. The Business Rules Builder is designed to make it easier for staff to report.
Attach records
This component enables you to upload or link related records, such as correspondence, emails, photographs relating to the non-conformance.
- Record name - When naming the record ensure the description is meaningful and easily found when searching. For instance, 'Email from Pharmacy re. delivery timeframes for medical consumables'.
- File or Link - click on Choose file and navigate to the record on your computer. If you have links enabled, toggle to change the control to add a URL to the record. The URL must be a in a web (http://) or Microsoft Sharepoint format (https://<company>.sharepoint.com/...).
- Viewing permissions - only users with permissions to see the item will be able to see attached records.
Submit/save the form
The green Submit button will save the form and progress the item to the next stage of the workflow.
Select Create a related item if you want to create a separate linked item on another register when you save the form. A link between the two items will be displayed in each item's System event history. This may be useful when you want to suggest an improvement in response to the non-conformance.